Do you believe in miracles? We sure do! In fact, we’re not just believers; we’re creating a miracle, and we want YOU to be a part of it.
Imagine a child reaching developmental milestones – crawling, saying ‘mama’ and ‘dada’ – only to have these abilities taken away before their second birthday. That’s the cruel reality of INAD/PLAN. In their last years of life, children cannot communicate, walk, sit up, or even eat on their own. INAD/PLAN is a devastating disease that takes away all a child can do, and when there’s nothing left, it takes the child, too. Sadly, most children don’t make it to their 10th birthday.
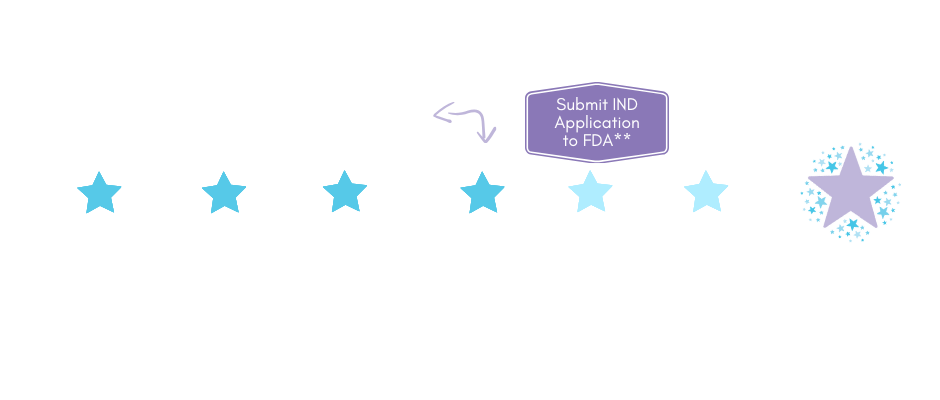
But there is hope. Scientists agree that gene therapy holds promise for treating INAD/PLAN. It has the potential to spare future families from unimaginable pain, as well as provide children like Sage, Ariya, Adrien, Zaidee, and Rex a real shot at fighting back. But we must move fast – the sooner kids are treated, the more likely they will have favorable outcomes.