INAD stands for Infantile Neuroaxonal Dystrophy otherwise known as PLAN (phospholipase associated neurodegeneration). INAD is a disorder that falls under the umbrella of NBIA (neurodegenerative brain iron accumulation).
Symptoms
The symptoms of INAD usually start to appear between the ages of 6 months and 2 years. A common pattern in young children is loss of previously acquired skills, mental and physical ability and progression of the disease over time. Many children experience delayed/difficulty walking, loss of neck control and low muscle tone in the trunk, which occurs early on. Eventually, muscle tightness and weakness in both arms and legs will follow.
Overactive reflexes is seen at the beginning of the disease and the absence of reflexes is seen later as the disease progresses.
Strabismus (crossed eyes) & nystagmus (involuntary eye movements) are often one of the very first indications of INAD, followed by optic atrophy (deterioration of the nerve that connects the eye to the brain).
Due to an impairment of the function of cranial nerves (Bulbar dysfunction), children affected by INAD often have speech problems, Dysarthria (poor articulation or slurring), Dysphonia (defective use of the voice), Dysphasia (difficulty in using or understanding words).
This leads to nutritional problems such as Dysphagia (difficulty swallowing), difficulty chewing, choking on liquids and nasal regurgitation. Due to these challenges of eating and drinking, children are often put on feeding tubes.
Some children experience seizures in early stages some may experience seizures as the disease progresses.
Prevalence
Infantile Neuroaxonal Dystrophy is an ultra-rare disorder. Its specific incidence is unknown, however it is estimated that INAD affects approximately 150 children worldwide. A diagnosis of INAD can now be confirmed through genetic testing of the PLA2G6 gene.
Causes & Inheritance Pattern
The human body is made up of millions of cells, and inside every cell there is a structure called DNA, which has detailed instructions on how all the parts of the body are put together and how they work. DNA is then further broken down into chromosomes. Humans typically have 46 total chromosomes that are organized in 23 pairs, there are two copies of each chromosome as we receive one set of 23 chromosomes from our mother and the other set of 23 chromosomes from our father.
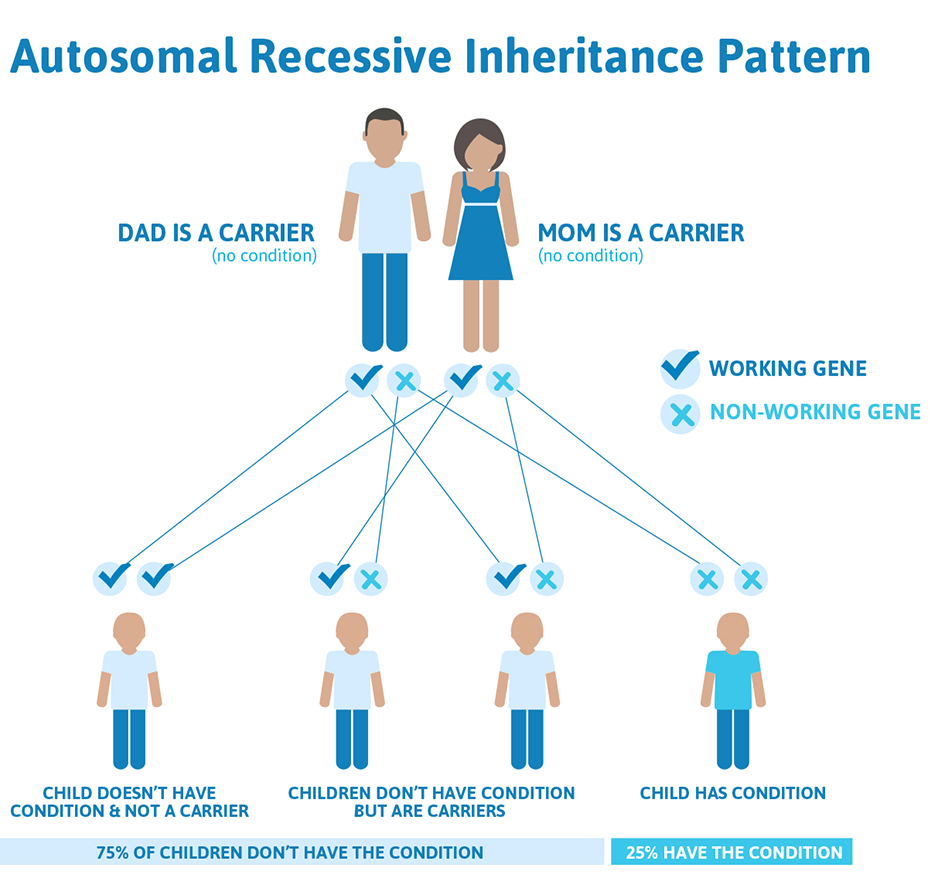
Chromosomes 1-22 are called autosomes, INAD has an autosomal recessive inheritence pattern, meaning that each parent is a carrier of the PLA2G6 mutation but do not show any effects of it. Since the sex chromosome, chromosome 23 is not involved, males and females are equally likely to inherit the mutated gene.
In a standard pregnancy, there is a 25% chance that carrier parents will pass on their recessive PLA2G6 gene and have a child with INAD, a 50% chance that the child will be a carrier like his/her parents, and a 25% chance that the child will not have INAD or be a carrier.
Diagnosis
In the past, an MRI of the brain and an ophthalmologic exam are key tests used to establish the clinical symptoms of INAD. An MRI can allow doctors to look out for cerebellar changes such as atrophy (degeneration of the cerebellum) and cerebellar hyperintensity (brightness) or hypointensity (darkness) in the globus pallidus, either of which could indicate brain iron accumulation. However, not all children with INAD have iron accumulation in the brain.
A diagnosis of INAD can now be confirmed through genetic testing of the PLA2G6 gene to find two gene mutations or through whole exome sequencing where all 20,000 genes are analyzed.
Progression and Treatment
The progression of INAD is usually rapid after the initial onset of symptoms. Many affected children never learn to walk or lose this ability shortly after learning it. During the last stages of the disease, severe spasticity (tight or stiff muscles), progressive cognitive decline and problems with vision have a large impact on daily life. Unfortunately, many children with INAD do not live beyond age 10, but some do survive into their teens or later ages. Supportive care and symptom management can lead to a longer life span by reducing the risk of infections and other complications.
While there is currently no standard treatment for INAD, there are mouse models of INAD and also models of INAD using cells in a test tube. In both models, the polyunsaturated fatty acid, docosahexanoic acid (DHA) has been proven to be beneficial.